10th Congress of the European Federation of Colposcopy (EFC)
Procare Health was honoured to participate as a Platinum Sponsor at the 10th Congress of the European Federation of Colposcopy (EFC), held in Riga from 18-21 September 2024. This prestigious event, focused on working towards the elimination of cervical and other HPV-related cancers, was the ideal setting for the presentation of the results of the new PALOMA 2 clinical trial.
A new randomised, multicentre, prospective, controlled clinical trial versus standard clinical practice. In it, it has been demonstrated with statistically significant results that the use of Papilocare® Vaginal Gel increases the clearance of HR-HPV compared to the 6-month watchful waiting. These data confirm and reinforce the data observed in the PALOMA 1 clinical trial.
We invite you to discover the details of this important study in the recording of the symposium that took place at the EFC congress:
In addition, 3 oral communications on the efficacy of Papilocare® Vaginal Gel were accepted and presented during the congress. The abstracts are available here:
Abstracts EFC 2024
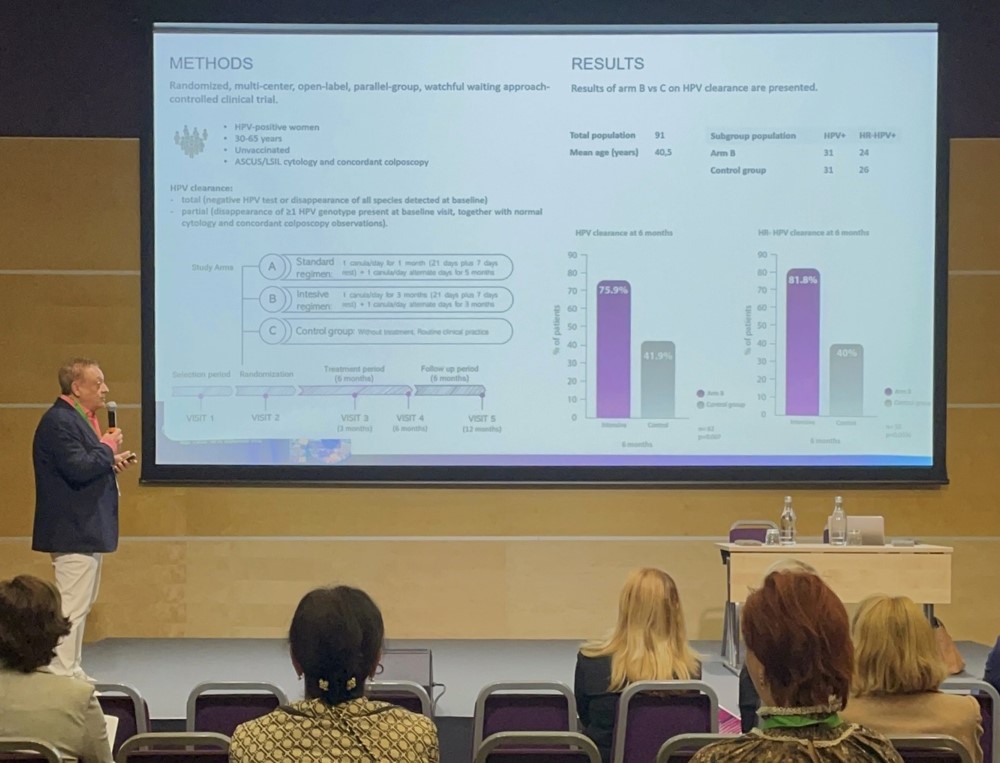
Efficacy of intensive regimen of a multi-ingredient Coriolus versicolor-based vaginal gel in increasing HPV clearance: Results from the PALOMA Clinical Trial – Dr. Luis Serrano
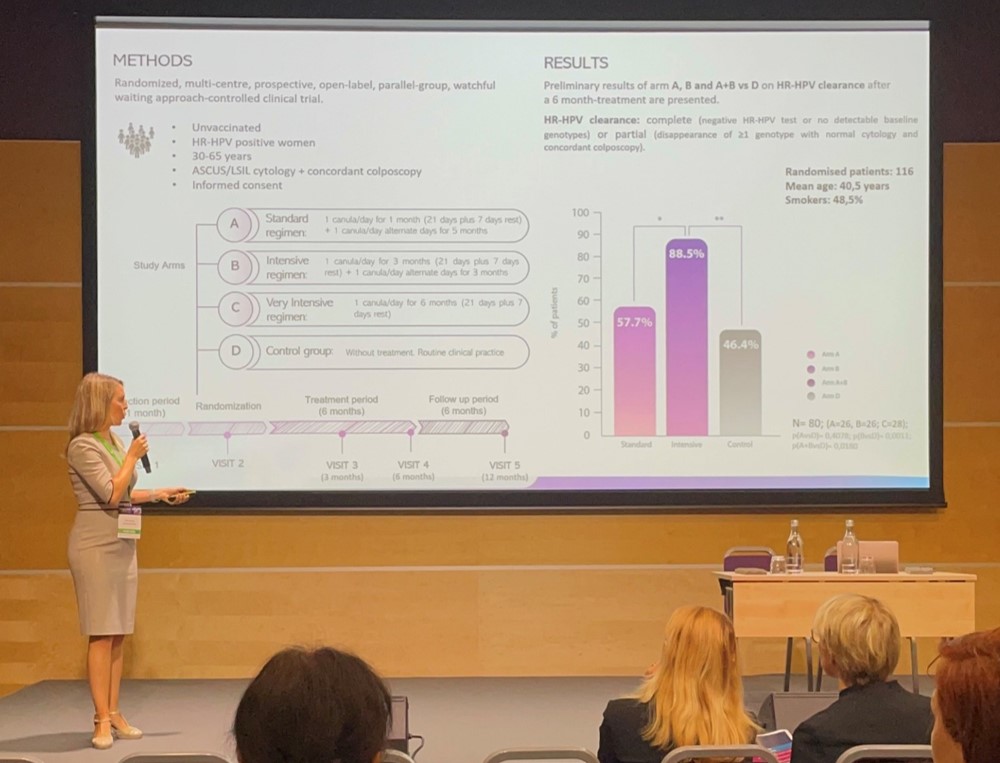
Efficacy of intensive regimen of a multi-ingredient Coriolus versicolor-based vaginal gel on high-risk HPV clearance: Preliminary results from the PALOMA 2 Clinical Trial – Dra. Patricia Sanmartín
Efficacy of intensive regimen of a multi-ingredient Coriolus versicolor-based vaginal gel (Papilocare®) in HR-HPV clearance: Preliminary pooled results from the PALOMA 1 and PALOMA 2 Clinical Trials – Dr. Luis Serrano
